Introduction: Regulatory T cells (Tregs), a highly immunosuppressive subset of CD4 T cells, are enriched in B-cell non-Hodgkin lymphoma (NHL) and constitute a barrier to potent antitumor immune responses. Despite extensive studies, the significance of tumor-infiltrating Tregs on disease outcome is unclear and while Tregs may express co-inhibitory and co-stimulatory receptors, the role of intratumoral Tregs in the context of immune checkpoint therapy remains elusive. Emerging evidence suggests heterogeneity among Tregs and their suppressive capacities in cancer, emphasizing the need for additional markers to identify highly suppressive Tregs. Therefore, an in-depth characterization of Treg heterogeneity in NHL could provide important insight into the disease pathogenesis and have implications for rational drug design.
Methods: Expression of checkpoint receptors in Tregs was characterized by fluorescence flow cytometry and mass cytometry analysis of single-cell suspensions from diffuse large B-cell lymphoma (DLBCL; n = 16), follicular lymphoma (FL; n = 8), mantle cell lymphoma (MCL; n = 10), marginal zone lymphoma (MZL; n = 2), chronic lymphocytic lymphoma (CLL; n = 7), as well as tonsils (n = 8) and peripheral blood (n = 4) from healthy donors. Functional characterization of intratumoral Tregs was performed by a proliferation assay using FACS-sorted Tregs as suppressor cells and autologous CellTrace Violet-labelled T effector cells as responder cells. Single-cell RNA sequencing (scRNA-seq) was performed on FACS-sorted CD4 T cells from 3 DLBCL, 3 FL and 3 healthy donor tonsils using the 10X Genomics single cell 5' based library construction and VDJ libraries for TCR-sequencing. Additionally, for simultaneous profiling of phenotypic features with the mRNA expression in single cells, Cellular Indexing of Transcriptomes and Epitopes by Sequencing (CITEseq) was applied. The Treg compartment was characterized by clustering into distinct transcriptional Treg states and differential expression of marker genes.
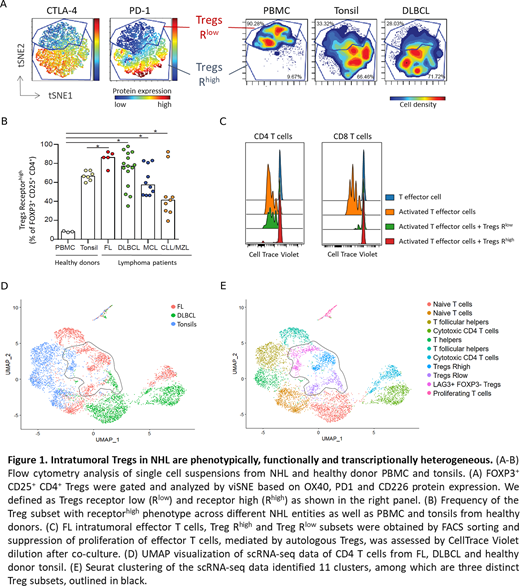
Results: TIGIT and CTLA-4 were identified as common markers of intratumoral Tregs, in addition to FOXP3 and CD25. Unsupervised computational analysis revealed two distinct Treg subsets, based on contrasting expression of PD-1, OX40, CD226 and ICOS (Figure 1A). One subset displayed a checkpoint receptorlow phenotype that corresponded to peripheral blood Tregs. The second subset had a checkpoint receptorhigh phenotype with elevated levels of PD-1, OX40, ICOS, TIGIT, CTLA-4 and increased levels of activation markers CD28, CD69 and CD95/Fas. The frequency of checkpoint receptorhigh Tregs was significantly increased in NHL tumors, compared to PBMCs and tonsils from healthy donors. FL tumors had the highest frequency of Tregs with receptorhigh phenotype among the NHL entities (median frequency of 86%, range 71-92%) and DLBCL had the highest donor-to-donor variation (median frequency of 77%, range 35-98%) (Figure 1B). This phenotypic heterogeneity of the Treg compartment reflected different suppressive capacities of the two subsets. Checkpoint receptorhigh Tregs were more potent mediators of immunosuppression in terms of suppressing the proliferation of autologous effector CD4 and CD8 T cells (Figure 1C). Furthermore, transcriptomic analysis of CD4 T cells by scRNA-seq and CITEseq revealed distinct transcriptomic signatures of the checkpoint receptorhigh and -receptorlow subsets. In addition, a third subset of Tregs, characterized by increased expression of LAG3 and immunosuppression-associated genes (CTLA-4, IL10, CD38, KLRB1) but lack of FOXP3, was identified (Figure 1D-E). Analysis of scTCR-sequences to compare TCR repertoires and to identify developmental trajectories will further add to our knowledge of intratumoral Tregs.
Conclusions: These results reveal heterogeneity within the Treg compartment in NHL based on expression of checkpoint receptors, transcriptional profiles and suppressive capacities. As intratumoral Treg phenotypes differ from peripheral blood Tregs, this presents new therapeutic opportunities. Specific targeting of intratumoral Tregs would lead to stronger antitumor effects while limiting immune-related adverse events. A deeper understanding of Treg heterogeneity within the tumor microenvironment could therefore open new paths for rational design of immune checkpoint therapy.
Kolstad:Merck: Research Funding; Nordic Nanovector: Membership on an entity's Board of Directors or advisory committees, Research Funding. Alizadeh:Janssen: Consultancy; Genentech: Consultancy; Pharmacyclics: Consultancy; Chugai: Consultancy; Celgene: Consultancy; Gilead: Consultancy; Roche: Consultancy; Pfizer: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal